Approach cures human genetic disease in vitro
Sunday, 31 May 2009
A study led by researchers at the Salk Institute for Biological Studies, has catapulted the field of regenerative medicine significantly forward, proving in principle that a human genetic disease can be cured using a combination of gene therapy and induced pluripotent stem (iPS) cell technology. The study, published in the May 31, 2009 early online edition of Nature, is a major milestone on the path from the laboratory to the clinic.
"It's been ten years since human stem cells were first cultured in a Petri dish," says the study's leader Juan-Carlos Izpisúa Belmonte, Ph.D., a professor in the Gene Expression Laboratory and director of the Center of Regenerative Medicine in Barcelona (CMRB), Spain.
"The hope in the field has always been that we'll be able to correct a disease genetically and then make iPS cells that differentiate into the type of tissue where the disease is manifested and bring it to clinic."
Although several studies have demonstrated the efficacy of the approach in mice, its feasibility in humans had not been established. The Salk study offers the first proof that this technology can work in human cells.
Belmonte's team, working with Salk colleague Inder Verma, Ph.D., a professor in the Laboratory of Genetics, and colleagues at the CMRB, and the CIEMAT in Madrid, Spain, decided to focus on Fanconi anaemia (FA), a genetic disorder responsible for a series of haematological abnormalities that impair the body's ability to fight infection, deliver oxygen, and clot blood. Caused by mutations in one of 13 Fanconi anaemia (FA) genes, the disease often leads to bone marrow failure, leukaemia, and other cancers. Even after receiving bone marrow transplants to correct the haematological problems, patients remain at high risk of developing cancer and other serious health conditions.
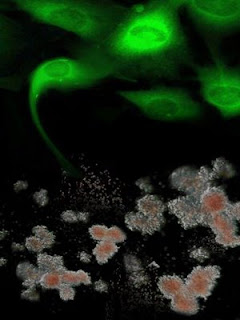 Shown in green are genetically-corrected fibroblasts from Fanconi anaemia patients are reprogrammed to generate induced pluripotent stem cells, which, in turn, can be differentiated into disease-free hematopoietic progenitors, capable of producing blood cells in vitro. Credit: Courtesy of Dr. Juan-Carlos Belmonte, Salk Institute for Biological Studies.
Shown in green are genetically-corrected fibroblasts from Fanconi anaemia patients are reprogrammed to generate induced pluripotent stem cells, which, in turn, can be differentiated into disease-free hematopoietic progenitors, capable of producing blood cells in vitro. Credit: Courtesy of Dr. Juan-Carlos Belmonte, Salk Institute for Biological Studies.
After taking hair or skin cells from patients with Fanconi anaemia, the investigators corrected the defective gene in the patients' cells using gene therapy techniques pioneered in Verma's laboratory. They then successfully reprogrammed the repaired cells into induced pluripotent stem (iPS) cells using a combination of transcription factors, Oct4, Sox2, Klf4 and c-Myc. The resulting FA-iPS cells were indistinguishable from human embryonic stem cells and iPS cells generated from healthy donors.
Since bone marrow failure as a result of the progressive decline in the numbers of functional hematopoietic stem cells is the most prominent feature of Fanconi anaemia, the researchers then tested whether patient-specific iPS cells could be used as a source for transplantable hematopoietic stem cells. They found that FA-iPS cells readily differentiated into hematopoietic progenitor cells primed to differentiate into healthy blood cells.
"We haven't cured a human being, but we have cured a cell," Belmonte explains.
"In theory we could transplant it into a human and cure the disease."
Although hurdles still loom before that theory can become practice — in particular, preventing the reprogrammed cells from inducing tumours — in coming months Belmonte and Verma will be exploring ways to overcome that and other obstacles. In April 2009, they received a $6.6 million from the California Institute Regenerative Medicine (CIRM) to pursue research aimed at translating basic science into clinical cures.
"If we can demonstrate that a combined iPS–gene therapy approach works in humans, then there is no limit to what we can do," says Verma.
About the Salk Institute for Biological Studies:
The Salk Institute for Biological Studies is one of the world's preeminent basic research institutions, where internationally renowned faculty probe fundamental life science questions in a unique, collaborative, and creative environment. Focused both on discovery and on mentoring future generations of researchers, Salk scientists make groundbreaking contributions to our understanding of cancer, aging, Alzheimer's, diabetes, and cardiovascular disorders by studying neuroscience, genetics, cell and plant biology, and related disciplines.
Faculty achievements have been recognized with numerous honours, including Nobel Prizes and memberships in the National Academy of Sciences. Founded in 1960 by polio vaccine pioneer Jonas Salk, M.D., the Institute is an independent non-profit organization and architectural landmark.
Reference:
Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells
Ángel Raya, Ignasi Rodríguez-Pizà, Guillermo Guenechea, Rita Vassena, Susana Navarro, María José Barrero, Antonella Consiglio, Maria Castellà, Paula Río, Eduard Sleep, Federico González, Gustavo Tiscornia, Elena Garreta, Trond Aasen, Anna Veiga, Inder M. Verma, Jordi Surrallés, Juan Bueren & Juan Carlos Izpisúa Belmonte
Nature advance online publication 31 May 2009, doi:10.1038/nature08129
.........
ZenMaster
For more on stem cells and cloning, go to CellNEWS at http://cellnews-blog.blogspot.com/ and http://www.geocities.com/giantfideli/index.html





No comments:
Post a Comment