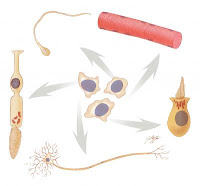
Own bone marrow stem cells increased circulation within the heart Monday, 30 March 2009 Patients treated with their own bone marrow stem cells after a heart attack experienced increased circulation within the heart, a study by Emory University School of Medicine physicians has found. Principal investigator Arshed Quyyumi, MD, professor of medicine at Emory University School of Medicine, presents the results Monday at the American College of Cardiology conference in Orlando. "These results show that treatment with a patient's own bone marrow stem cells has the potential to reduce long-term complications after a heart attack," Quyyumi says. "We are encouraged by these results and are planning to conduct a more extensive study." A severe enough heart attack can lead to remodelling of the heart muscle and increased risk of heart failure and other complications. Several groups of researchers around the world have reported clinical trials in which cells from the bone marrow are used to try to restore the heart's pumping power, with mixed results. This study was one of the first to use a preparation of bone marrow cells enriched for endothelial progenitor cells, which are thought to replenish the linings of blood vessels. Emory University, Vanderbilt University, The Lindner Research Center, Cincinnati, and Texas Heart Center in Houston participated. In the clinical trial, which began in June 2006, 31 patients were treated by angioplasty and stent placement after a heart attack. Within a week after their heart attacks, 16 of the patients had bone marrow cells infused into the coronary artery where a blockage caused the heart attack. Members of this group received three different amounts of magnetically sorted bone marrow cells (5, 10 and 15 million cells). The control group received standard medication only. No significant adverse events were reported. The patients will be followed for up to five years. Doctors assessed healing and remodelling of patients' hearts with nuclear (single photon emission computed tomography - SPECT) stress testing, magnetic resonance imaging, and echocardiography three months and six months later. Patients receiving higher doses of cells had greater improvement in blood flow within the heart than those patients treated with lower doses or those receiving medication alone. "This is critical information for future study design – the more cells a patient receives, the more beneficial effect we see in the heart," Quyyumi says. Higher doses of cells also appeared to provide some benefit in cardiac function, determined by measuring the percentage of blood pumped out with each heartbeat and tissue death due to loss of adequate blood supply, but these results were not considered significant statistically. ......... ZenMaster
For more on stem cells and cloning, go to CellNEWS at http://cellnews-blog.blogspot.com/ and http://www.geocities.com/giantfideli/index.html